COVID-19 PATIENT APP
In April 2020, Tempus rapidly launched a COVID-19 PCR testing business and a patient results app, moving from concept to thousands of users in just a few months. The project, Tempus’s first patient-facing experience, required building a clear, secure mobile app from scratch while navigating evolving regulations, tight technical constraints, and unprecedented timelines.
Working in a true war-room environment, the team coordinated across lab, engineering, legal, and scientific partners to define new standards for results delivery. The design work focused on rapid competitive research, tight technical scoping, and delivering a reliable, easy-to-understand experience for patients during a critical moment in the country.
THE CHALLENGE
A rapid-response project to design Tempus’s first patient-facing experience: a COVID-19 results app built from concept to launch in just a few months.
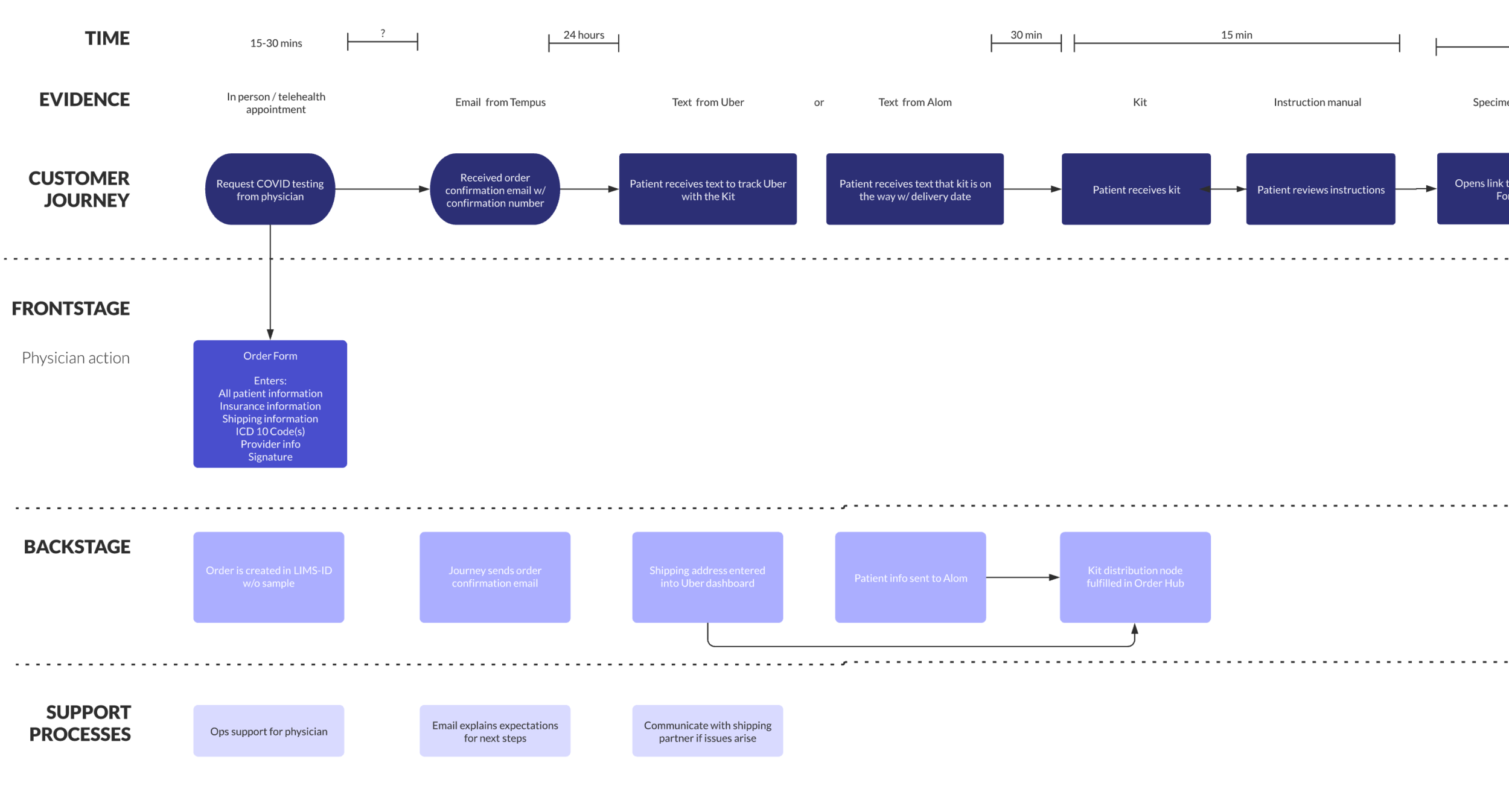
To drive clarity and alignment, I created journey maps, lightweight personas, and detailed technical specs with my engineering lead. I also built a series of narratives, docs, and slide decks to guide large, opinion-heavy scoping sessions.
In my joint Product Manager role, the early phase of the project focused on aligning our leadership team around the goals, scope, and metrics for the MVP. I worked closely with our CTO, CEO, Head of Security, and VP of Product—often multiple times a week—to rapidly iterate on requirements and validate our approach.
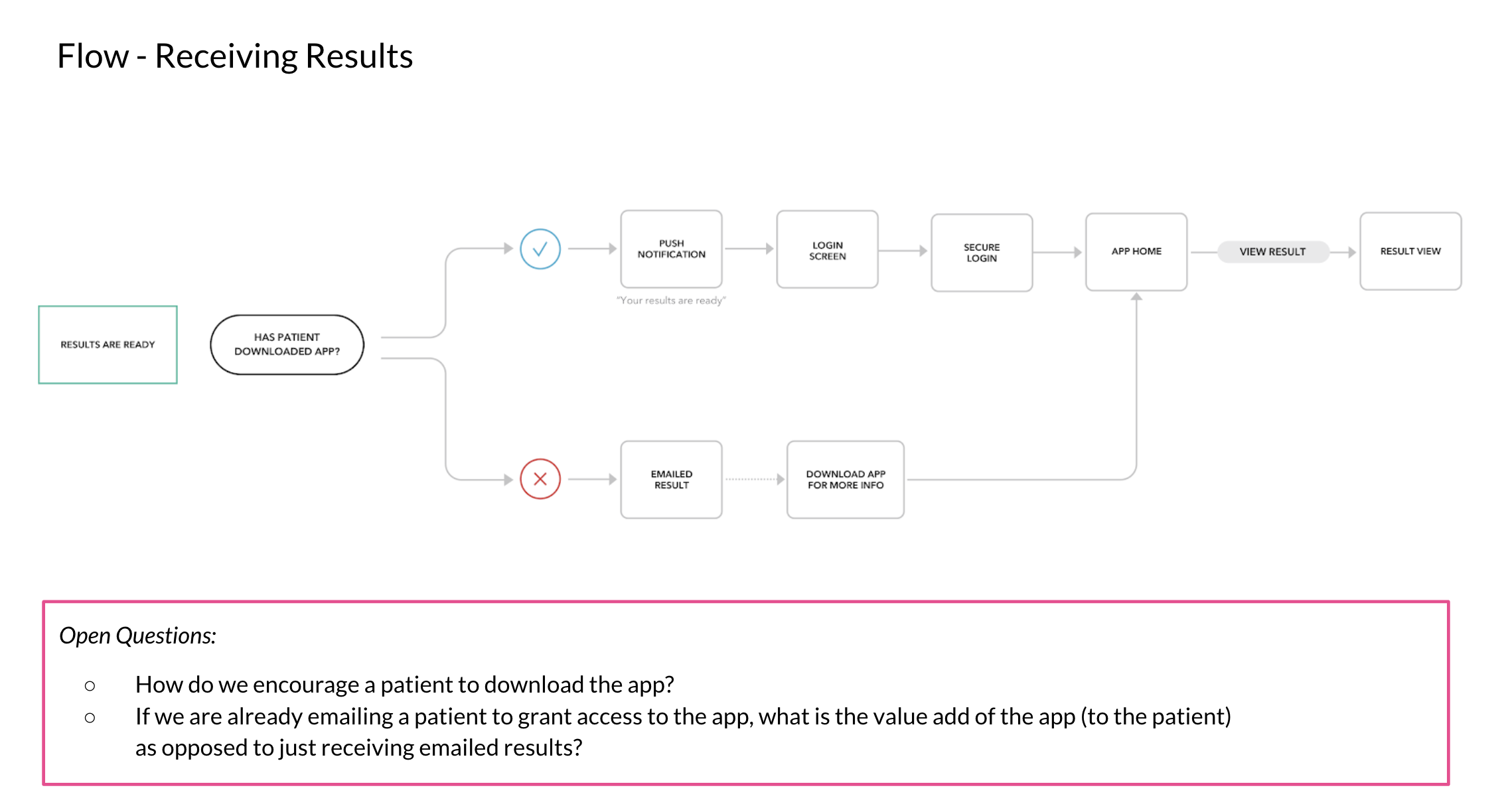
Why make a mobile app for result delivery?
An app allows a patient access to their results as soon as possible, without having to wait for a call from their doctor. With COVID, a few hours makes a big difference, especially at the start of the pandemic. We knew waiting for their results only caused our users more anxiety.
Clinical data! If patients consent via a form in the app, we can collect and de-identify their clinical data to support research efforts inside and outside of Tempus. The mobile experience lends itself well to a quick and easy onboarding process.
An app gets our foot in the door with a new user group, previously untouched by Tempus. Result delivery may be a one-time transactional relationship, but enables us to expand our direct-to-patient offering later on. We would now have an app ‘in their pockets’ to draw upon later.
This app was a great way to test the waters on how to work with patients directly, so we used it as a learning opportunity for other disease areas. Since the launch of this app, we have launched a mental health patient app and are broadening our touchpoints with oncology patients.
Who are the users?
At launch —
University students whose administrators partner with Tempus to test their student population // young, tech-savvy
Patients of major state-led healthcare systems and pharmacies, like CVS // broader demographic
A year in —
The above users (about 10-20K), plus…
Patients who ordered our home-collection kits // familiar with Tempus
Employees of companies doing regular testing // familiar with COVID-19 testing
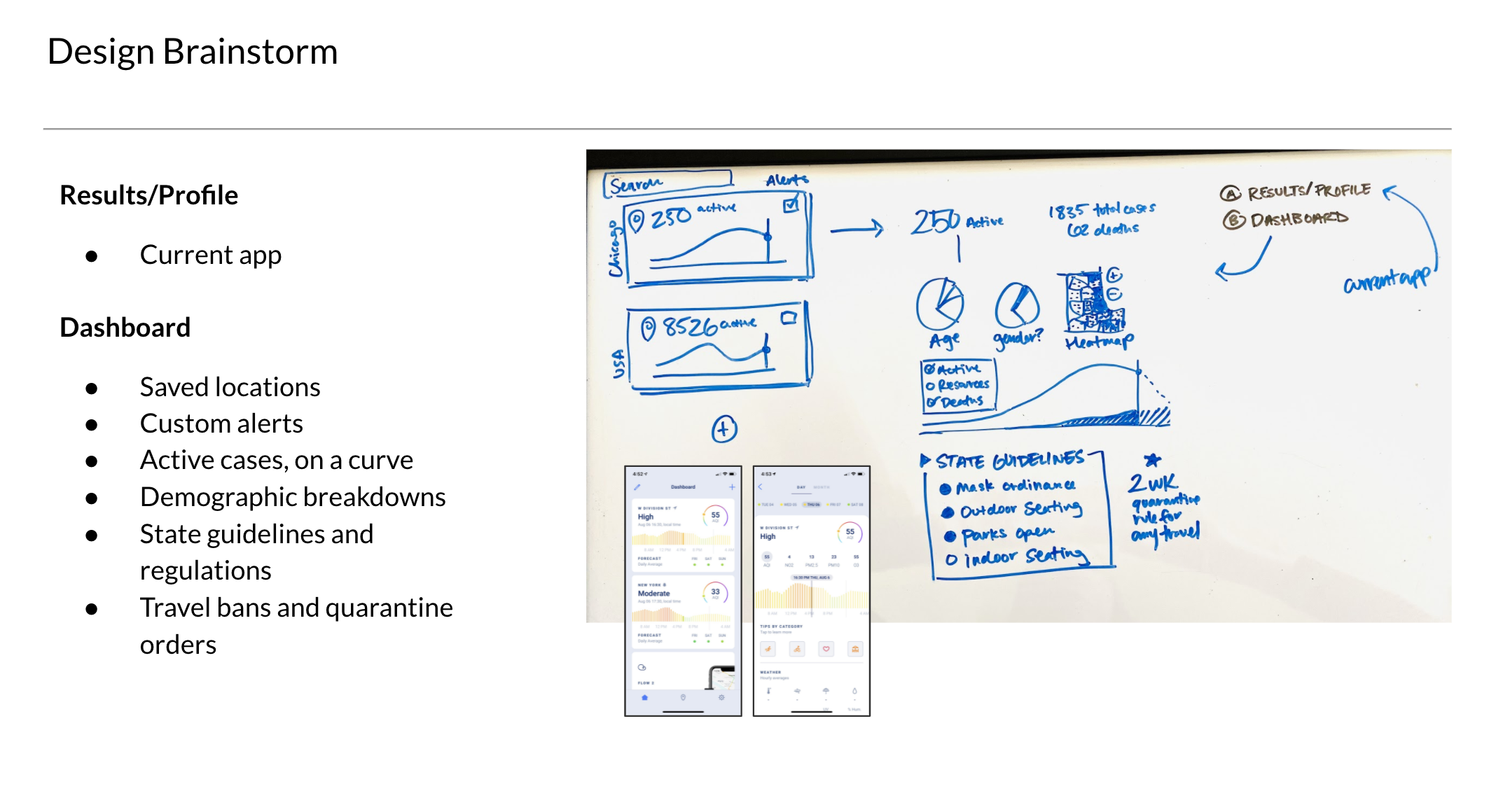
SCOPING
Our first launch had one goal: move fast. We paused all other work to ship a simple, secure solution we could quickly iterate on once real users were onboard.
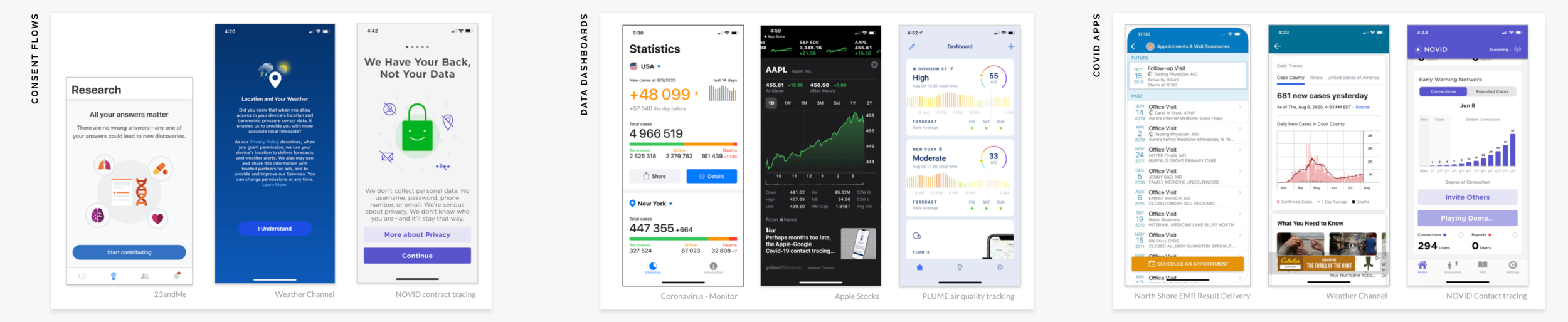
With only a few days to produce the initial concept, we prioritized competitive analysis over direct user research. We ran a rapid scan of existing apps and did quick guerilla research within Tempus to understand what people were using. From this, we identified three core patterns in the market.
Consent Flows | apps with an extensive flow for educating users about consent to share data
Data Dashboards | complex dashboards that display and compare trends over time
COVID Apps | existing COVID apps offering result delivery and trend viewing
MVP LAUNCH
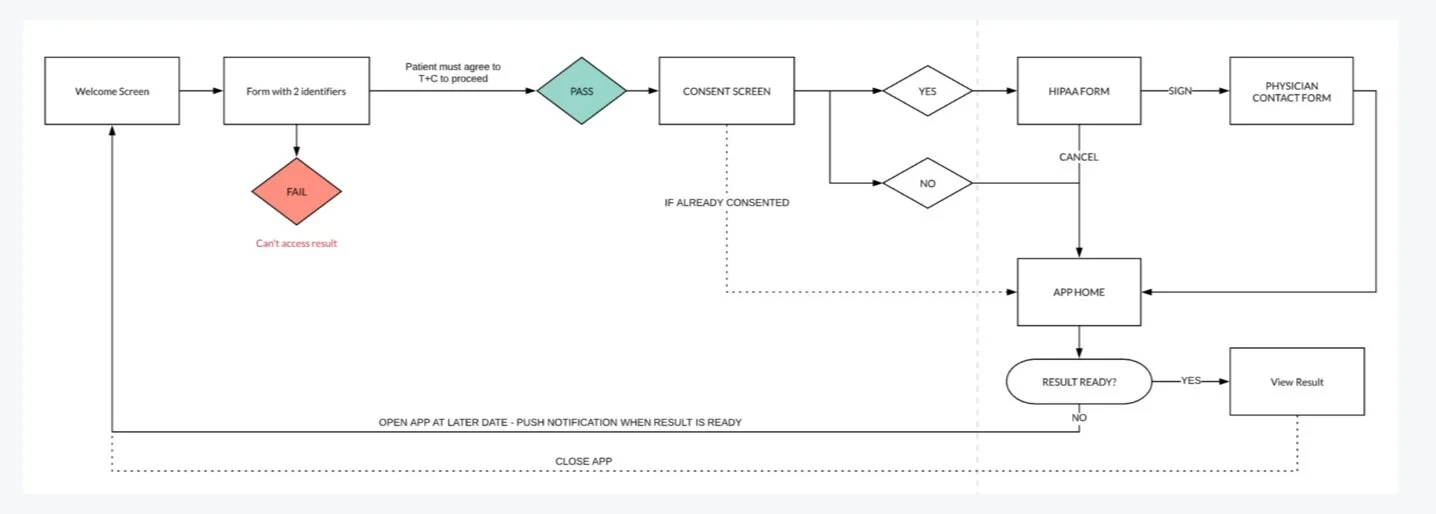
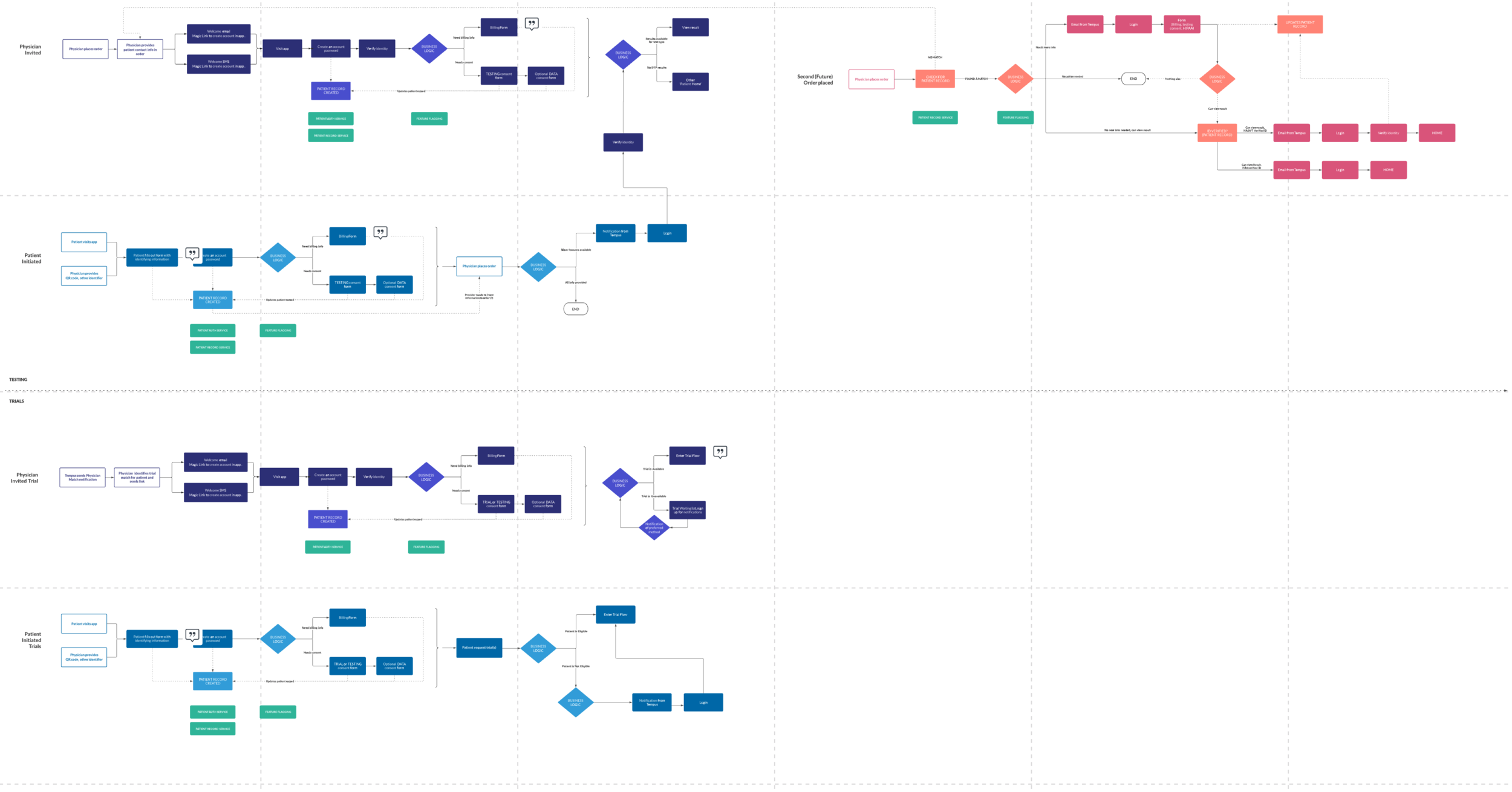
To move quickly, the initial release focused on only the most essential functionality. Priority one was a secure, low-friction entry into the app, balanced against strict technical, legal, and security requirements.
We worked closely with engineering and security through multiple review cycles to ensure patients could be confidently identified before releasing PHI. The MVP was intentionally scoped to:
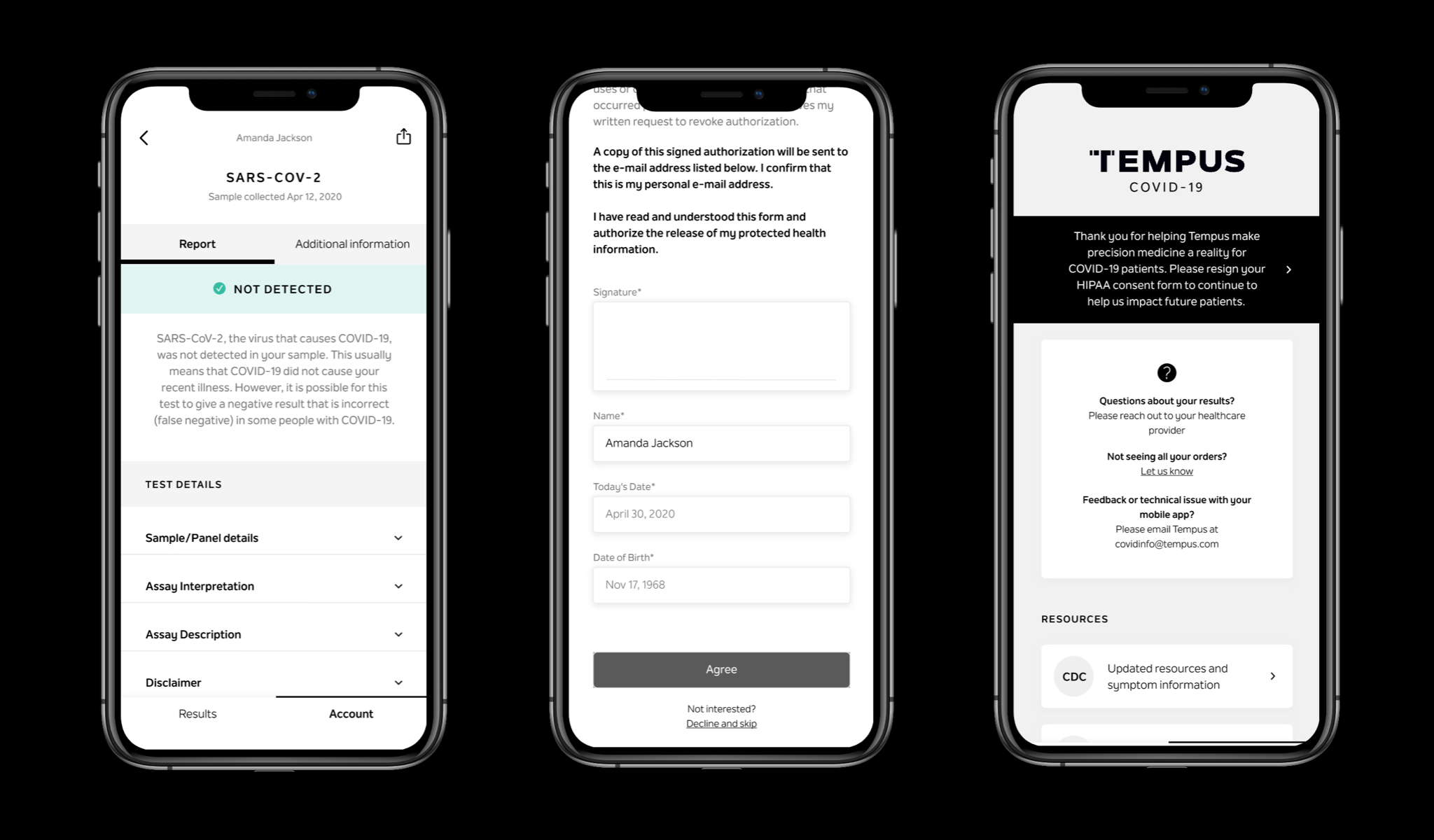
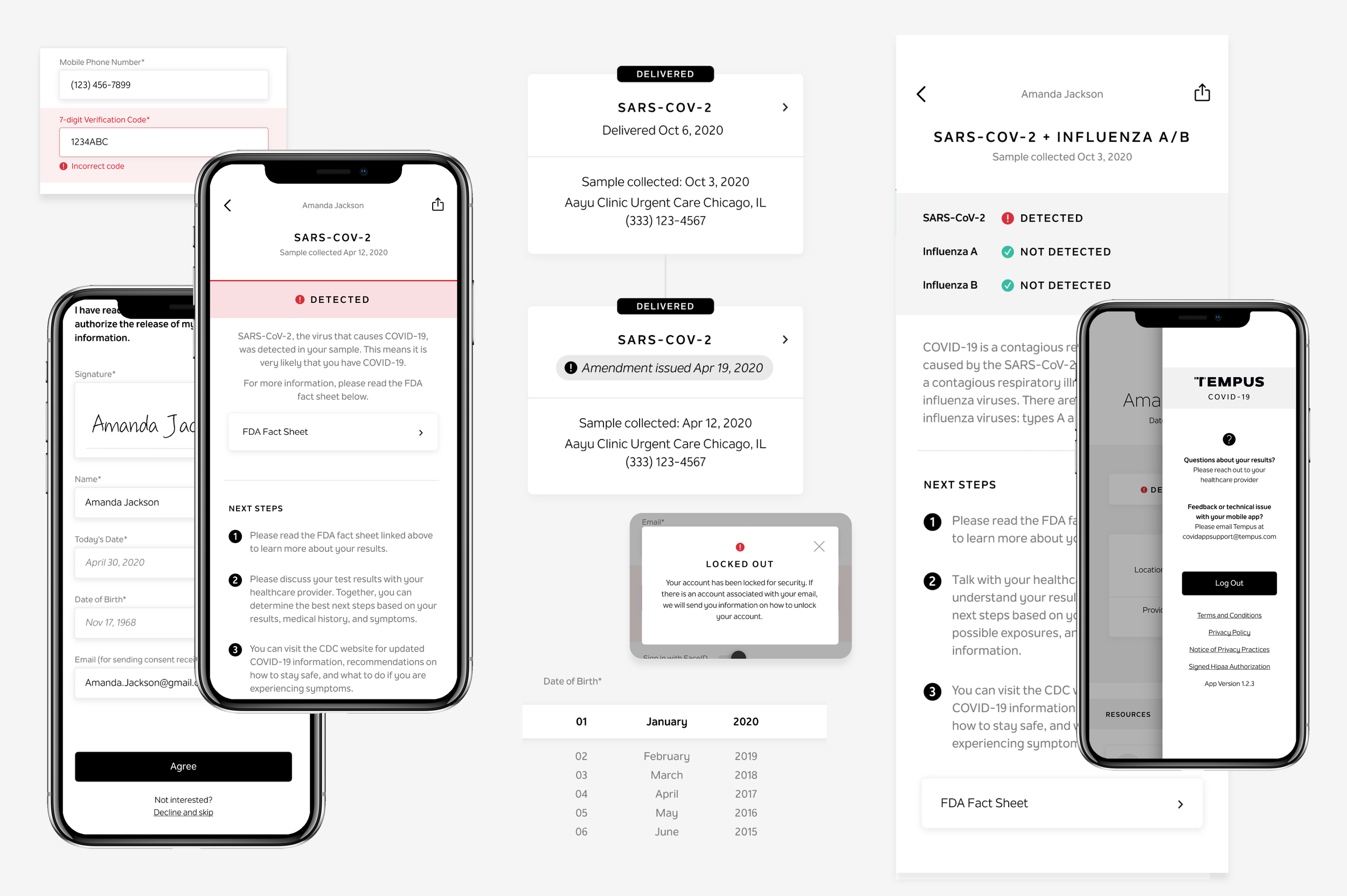
Secure account creation and authentication
Patient consent for clinical data collection
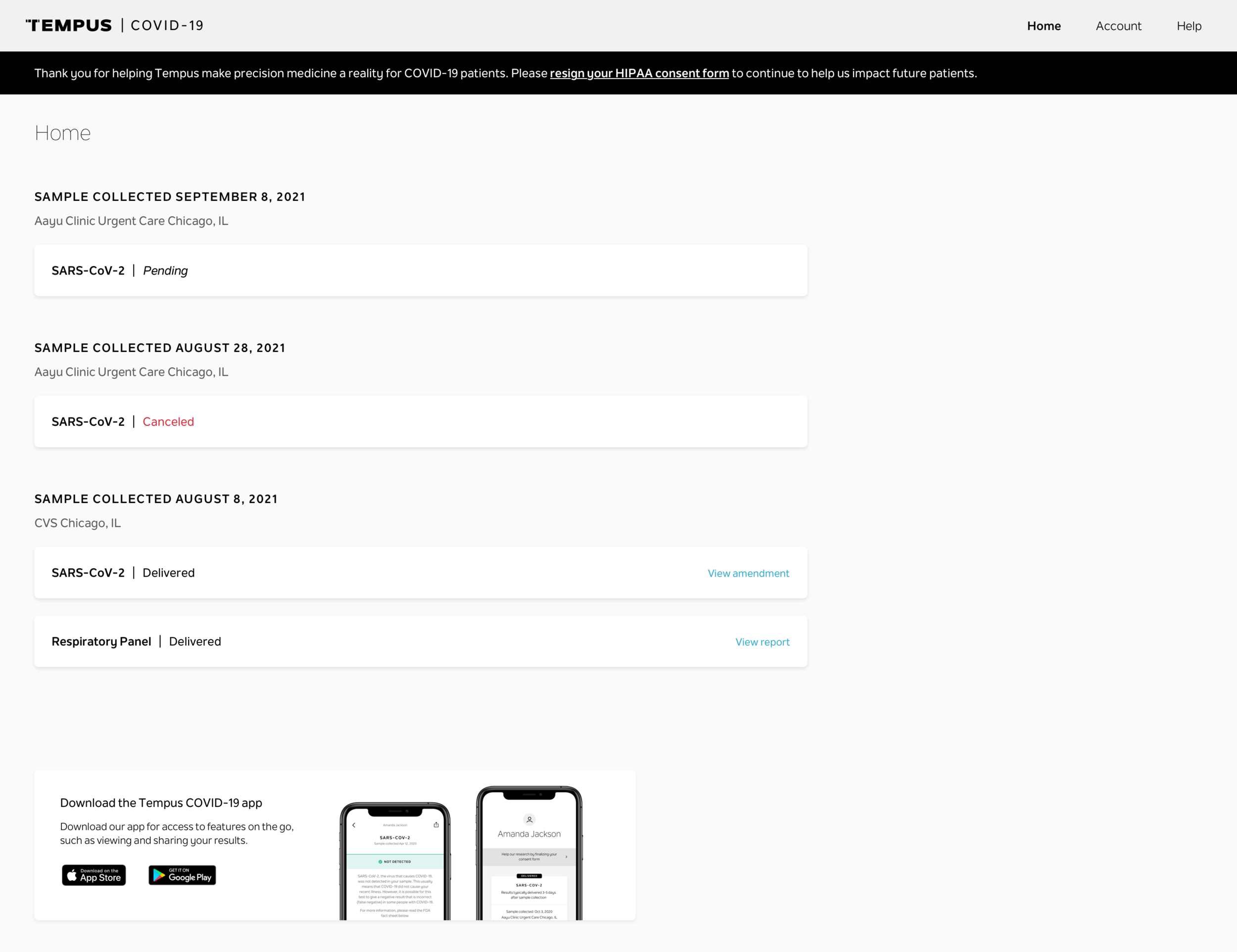
Test results and basic order status
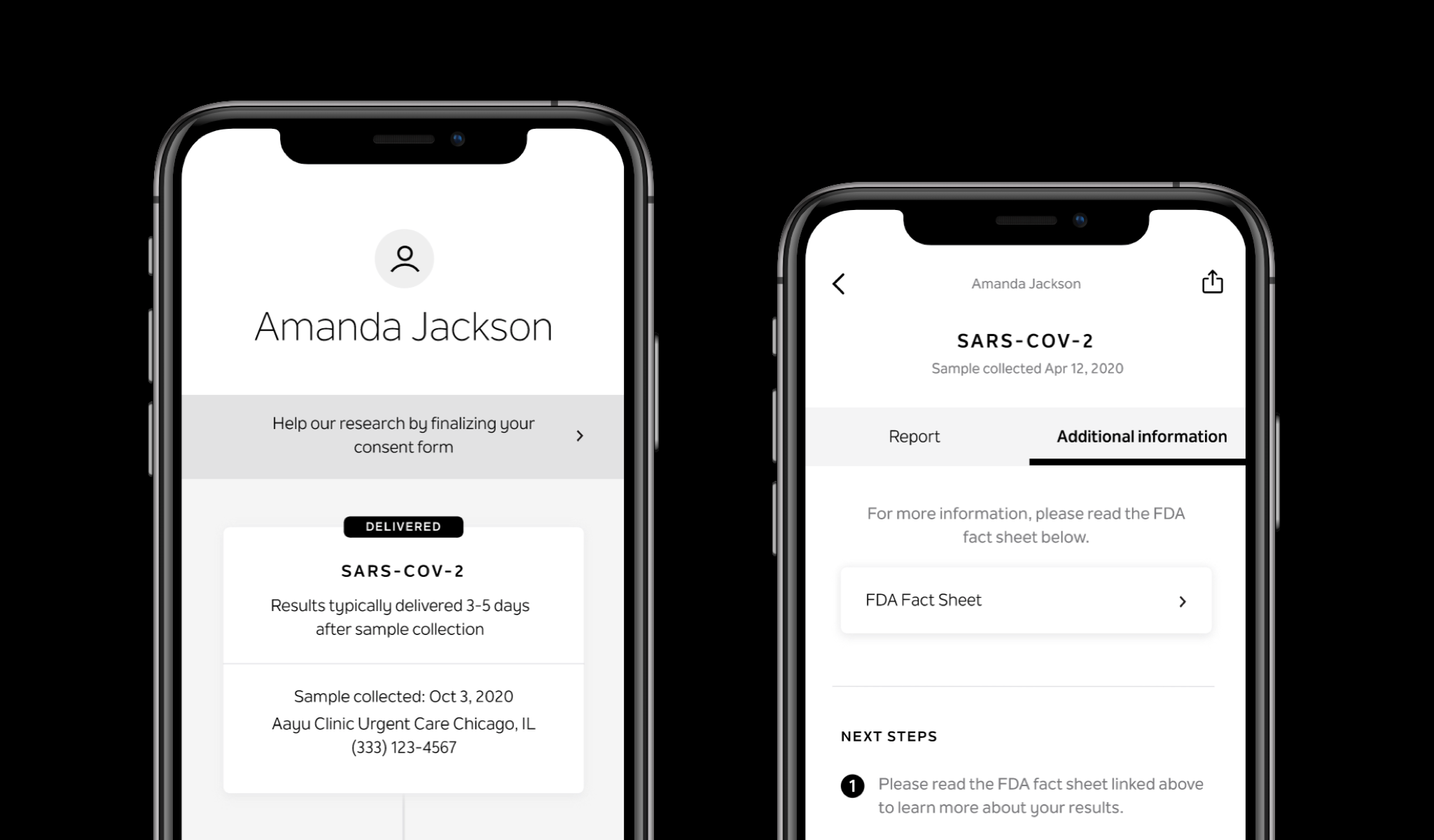
FDA-required patient resources
The app launched in September 2020—less than six months after the decision to support COVID-19 testing.
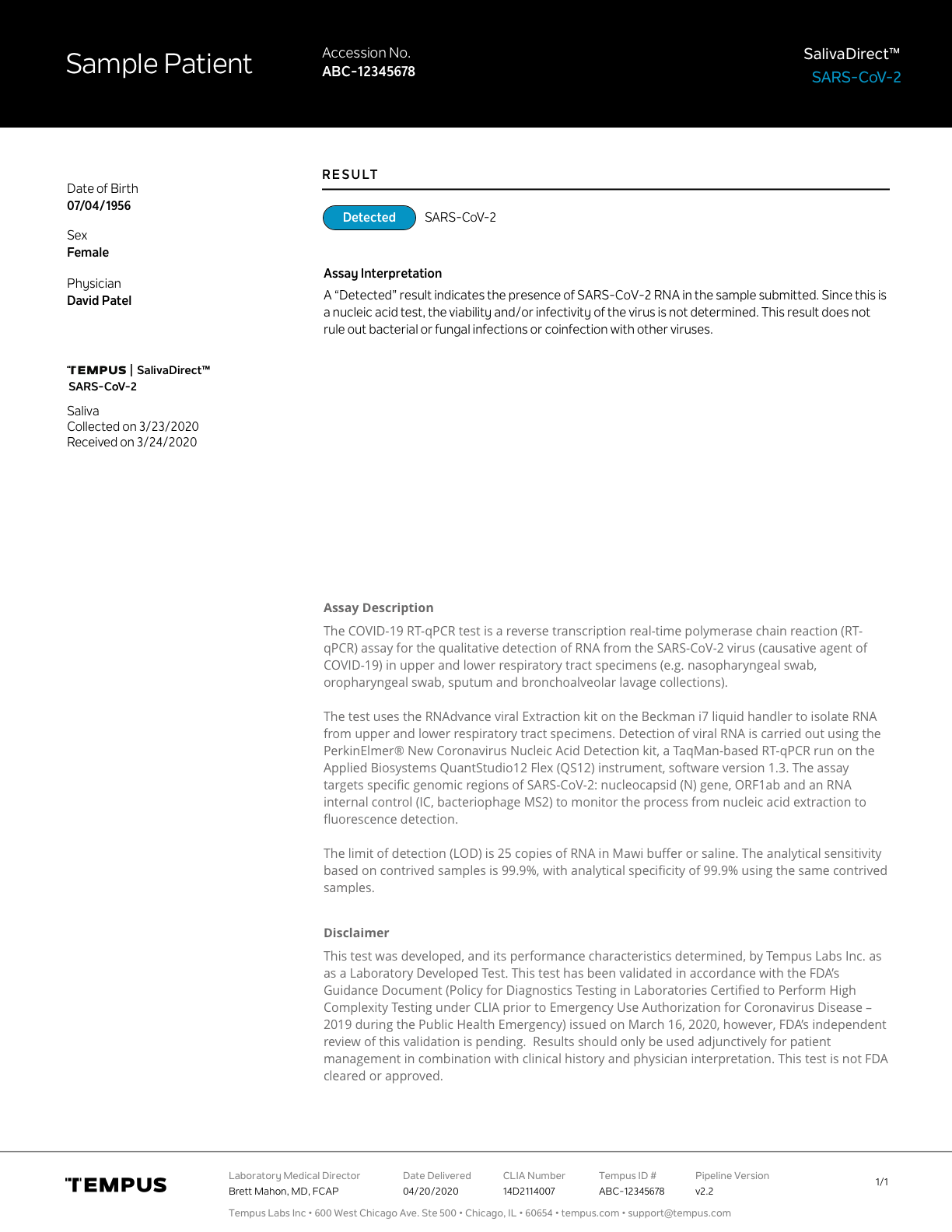
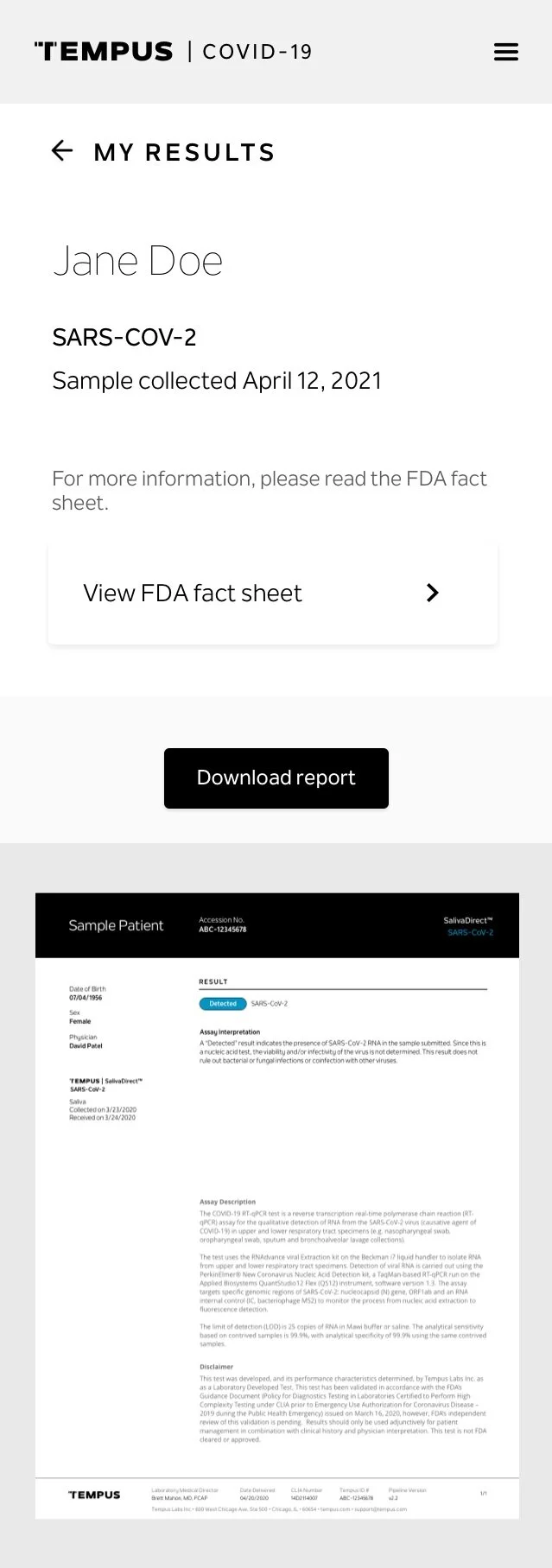
PATIENT REPORT DESIGN
I also designed the reports for our infectious disease assays, aligning them with Tempus’s existing report standards and design ecosystem. These reports were adapted for mobile-first delivery, with results dynamically rendered to improve clarity for patients, and later translated again to support web-based results delivery.
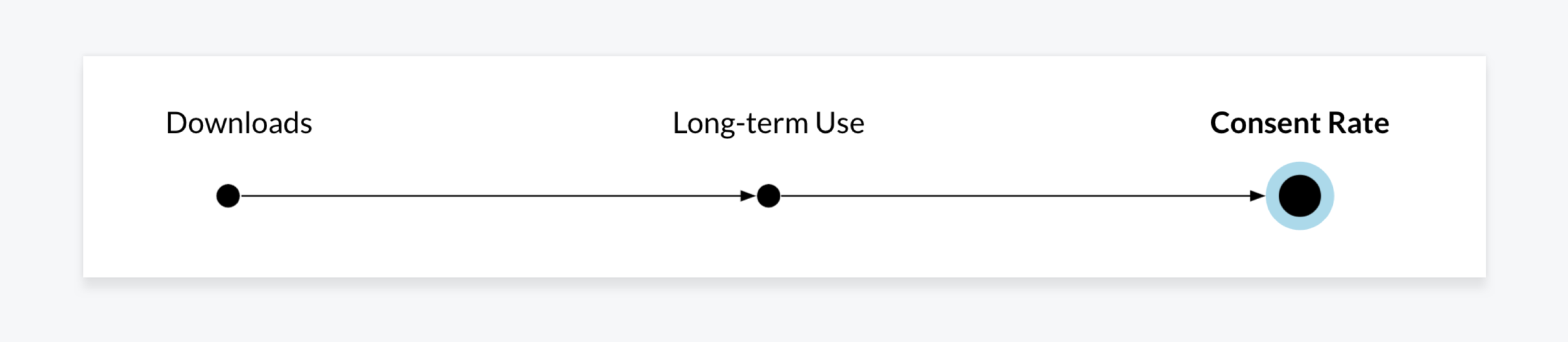
After launch, our primary goal shifted to increasing patient consent for clinical data collection. To support that, we needed to move beyond one-time results delivery and encourage repeat engagement. Success was no longer measured by app downloads, but by the number of consented patients.
The challenge was identifying where Tempus could offer unique value in an already crowded COVID app landscape. We explored several retention-focused strategies, using high-fidelity concepts to align stakeholders on a small set of directions worth pursuing.
POST-MVP
We narrowed our pitch to three concepts centered on delivering and collecting data. While we explored ideas like contact tracing, patient ordering, and symptom tracking, we ultimately focused on Tempus’s strongest differentiator: its data.
This direction was shaped through multiple review cycles with the CEO, CTO, and technical leadership, along with guidance from an in-house scientist specializing in patient-reported outcomes. With patient consent as our primary KPI, we used consent rate as a proxy for user trust and value. That metric launched strong and remained consistently high throughout the life of the app.
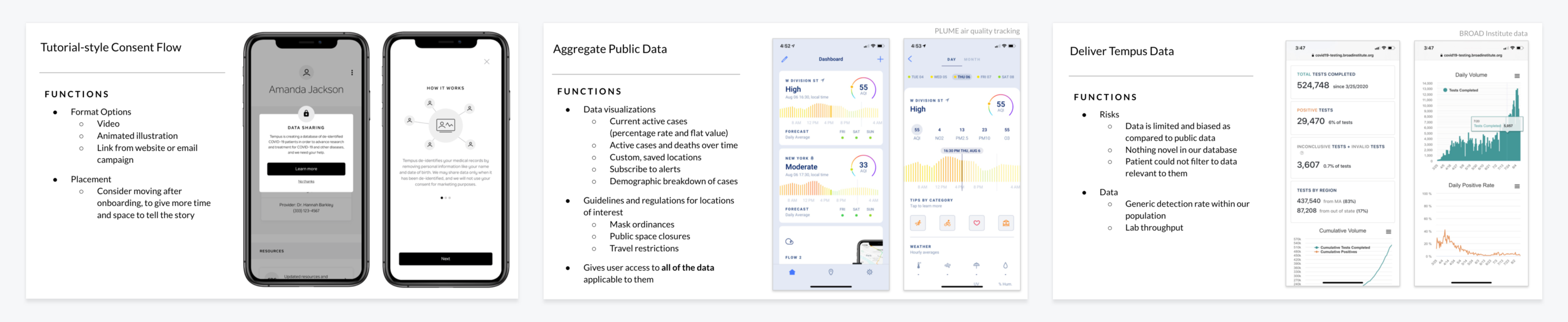
Tutorial-Style Consent Flow | reframe the way we ask for consent to increase transparency
Aggregate Public Data | connect to vast publicly-available datasets
Deliver Tempus Data | highlight findings from our own dataset in real time
USER TESTING
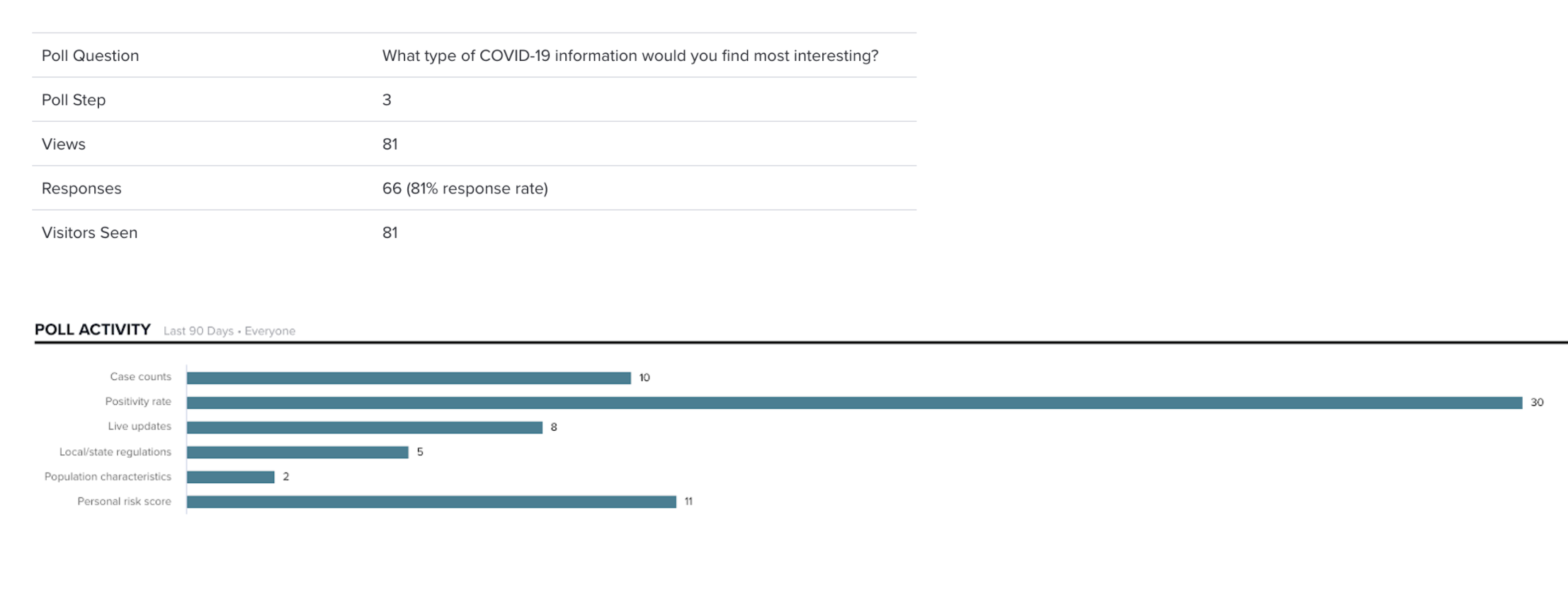
During development, we mapped all key user actions in Pendo to enable fast, data-driven insights. These metrics were essential for reporting launch success and measuring the impact of each feature release.
Pendo also gave us lightweight tools—like in-app announcements and surveys—without additional engineering effort. Together, these capabilities helped us better understand user behavior and informed future product decisions.
OUTCOMES
Tempus has delivered COVID-19 results directly to patients since September 2020, reaching ~2,000 users per week at peak. Post-launch, we iterated on onboarding to reduce Customer Support interventions while increasing activation and consent rates.
Based on direct partner and user feedback, we also introduced a mobile-friendly web flow that removed the need for an app download. This app allowed patients who would never download an app on their phone, or more importantly those without access to a personal device, to access to their COVID-19 results quickly.
Following the launch, my focus shifted to applying lessons from this work across other patient touchpoints at Tempus. Patient experiences varied widely by disease area, and I advocated for a funded discovery effort to address that inconsistency.
The project centers on auditing the end-to-end patient experience through interviews with operations and customer success teams, paired with journey and process mapping. In parallel, I worked with my PM to evaluate and scope technical and operational solutions for future rollout.